Personalised And Generalised Integrated Biomaterial Risk Assessment

Today, biomaterial-based solutions play an important role in clinical applications.
They can be implemented e.g. astissue repair scaffolds or replace elements of damaged body parts. As these biomaterials directly interact with the human body, unforseen adverse reactions can occur.
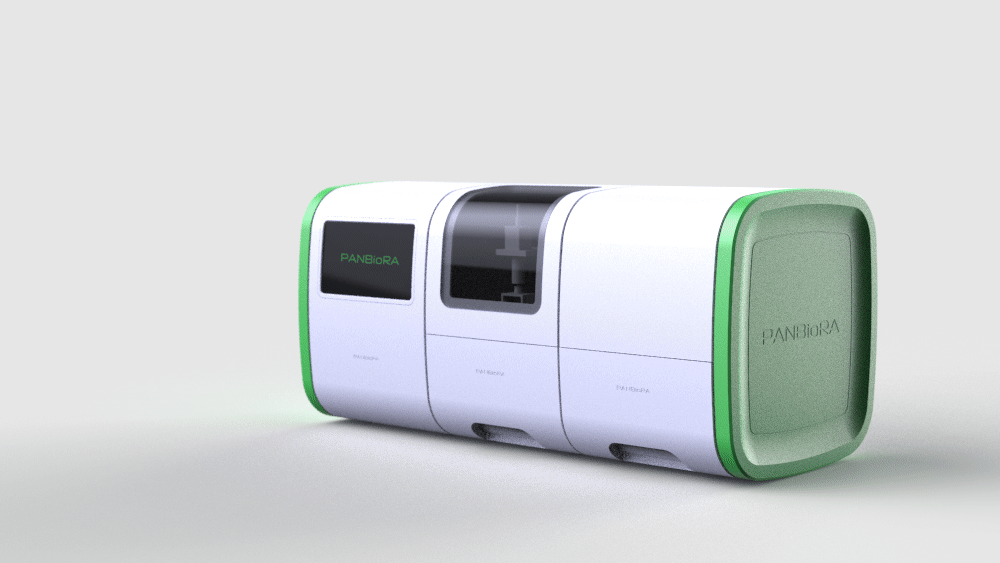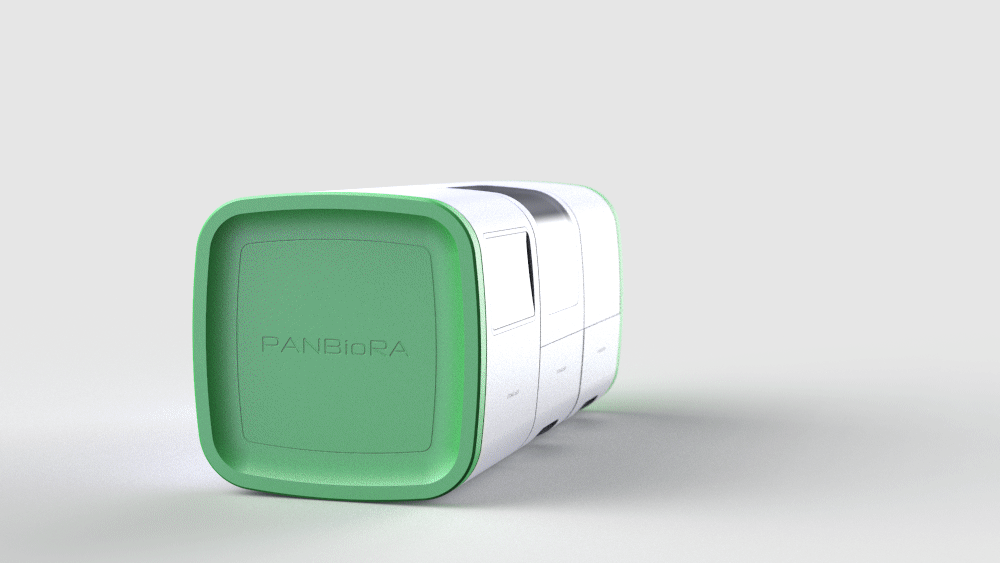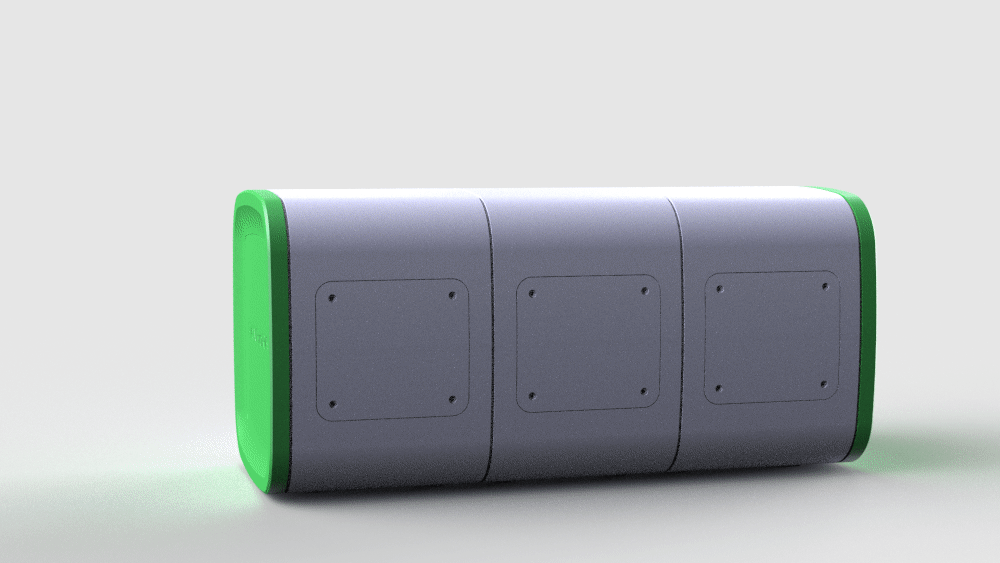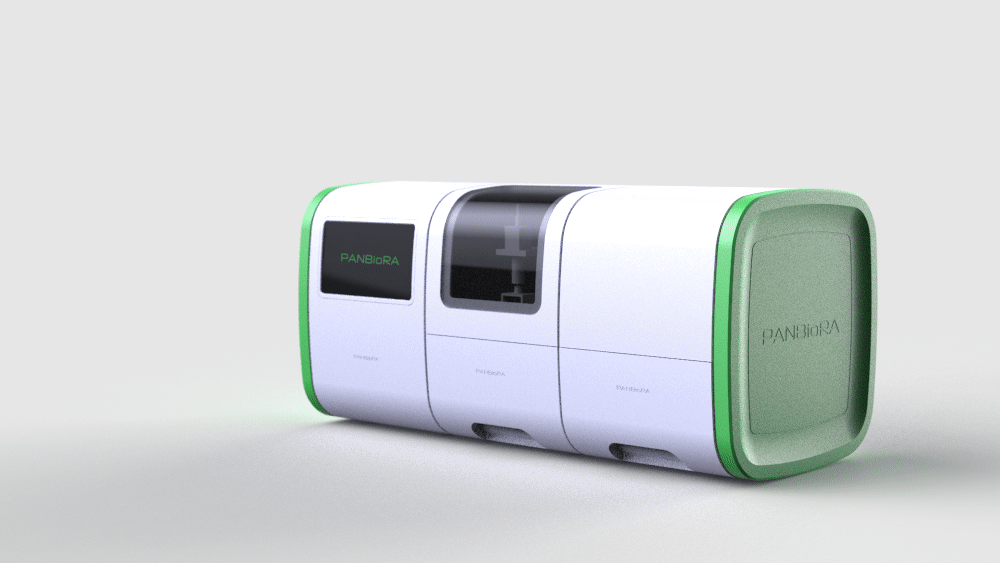
PANBioRA will develop a tool to better assess a patient-specific reaction to biomaterials and thus allow improved clinical outcomes.
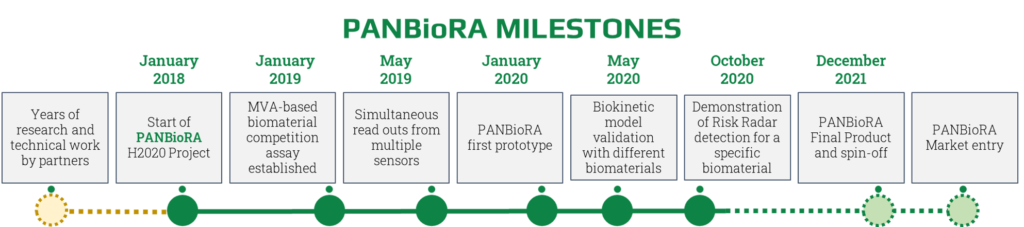
PANBioRA Timeline
What does PANBioRA provide?
The right solution at the right time
The complexity of the new generation of biomaterials and implants put a lot of pressure on the relevance of the current testing methods. In addition, there is a policy-level awareness of the need for new instrumentation. PANBioRA directly tackles the ongoing trends and needs of personalised medicine and point-of-care devices with an integrated multifunctional system with game-changer potential.
Antibody testing
Patient-specific interactions between biomaterials and the immune system will be assessed using the ground-breaking Mimotope Variation Analysis technology.
Biomaterial testing
Biochemical responses of cells to the presence of biomaterials will be monitored in real time and integrated biosensors. In addition, the project will quantify the risk of cyto- and genotoxicity of new materials and their degradation products using mini-microscopes.

Cell testing
Real-time electrochemical sensing will be used to determine the cellular response to a given biomaterial. A set of cytokines released to the extracellular environment will be used as biomarkers to assess the cell response to different biomaterials.

Organ on a chip
Respiratory epithelium, gut and liver tissues will be miniaturized into organoids on chip to allow the determination of possible systemic and target organ-specific effects in both healthy and disease conditions.

Simulations
Potential risks that are difficult to assess experimentally – such as explosion hazards and full-scale biomaterial/microbiota interactions – will be covered by simulations developed within the project.
Data analysis
The readouts of the modules will be fed into a model developed within PANBioRA using known biocompatible and hazardous materials to provide a quantitative risk assessment.

Risk rating
PANBioRA will develop a risk rating system that will display the suitability of the tested biomaterial using well characterized materials as reference.
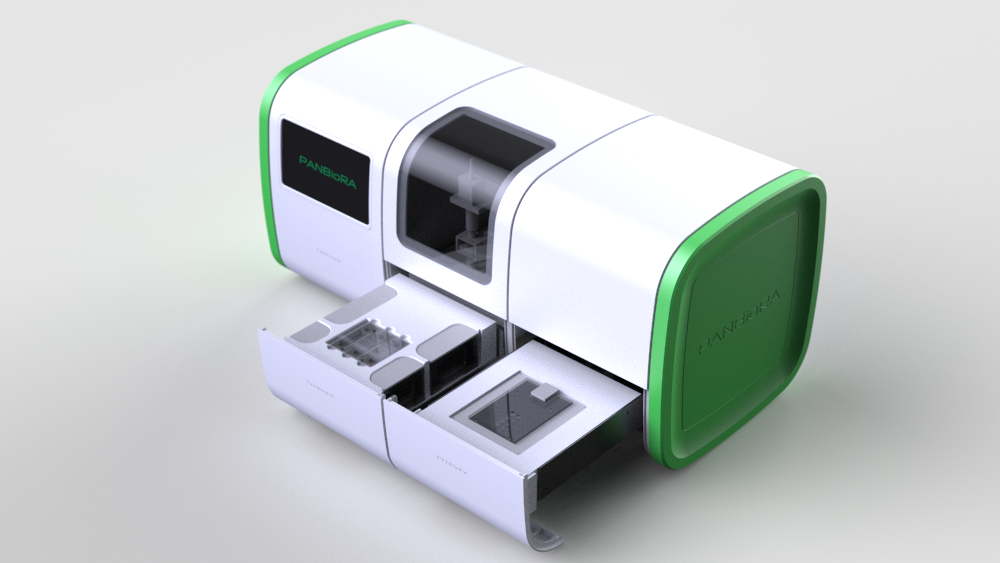
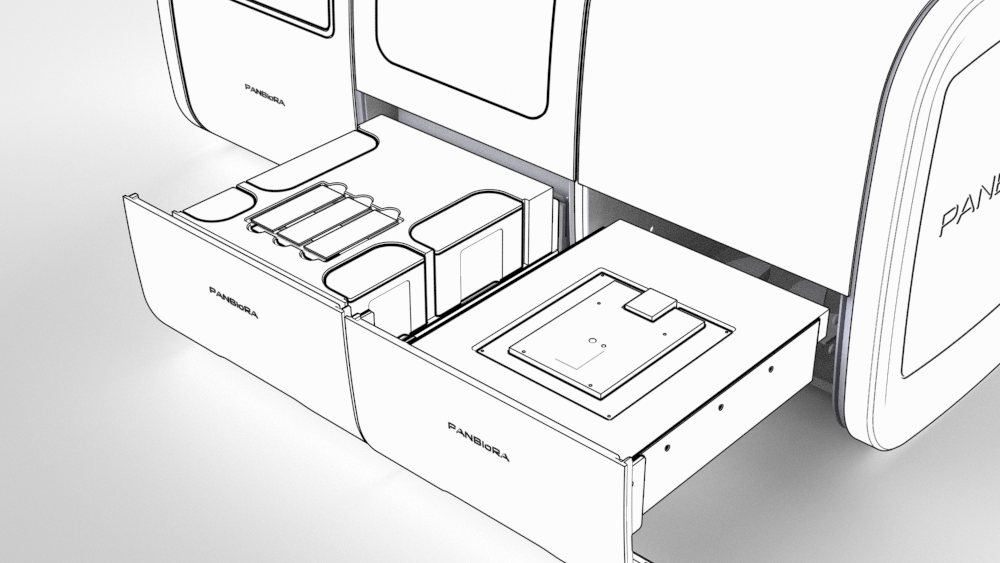

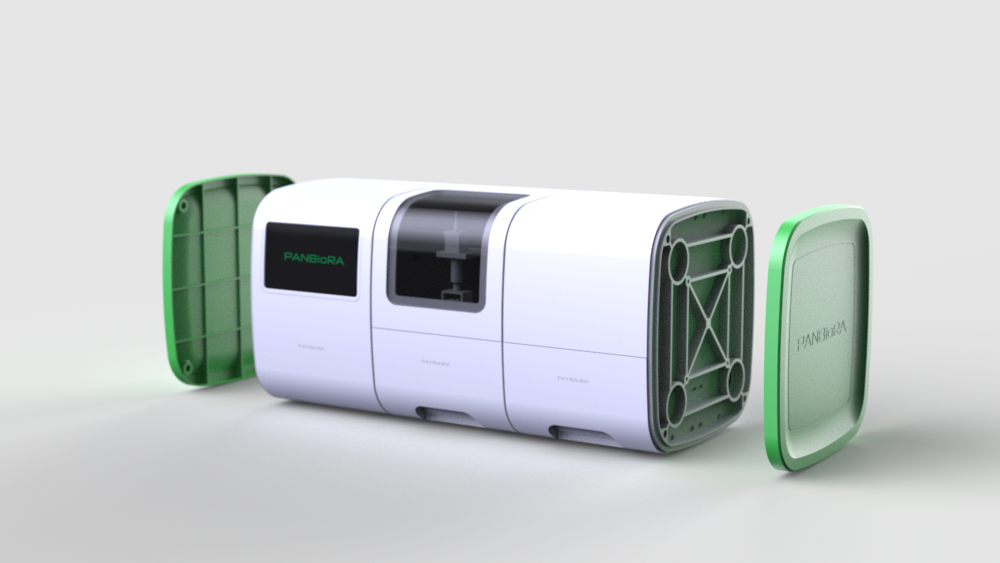

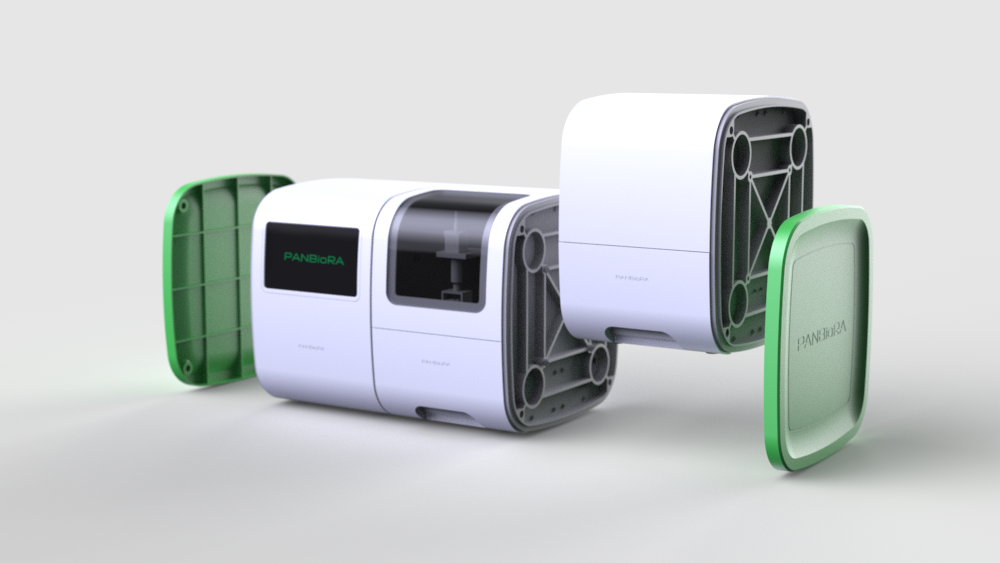
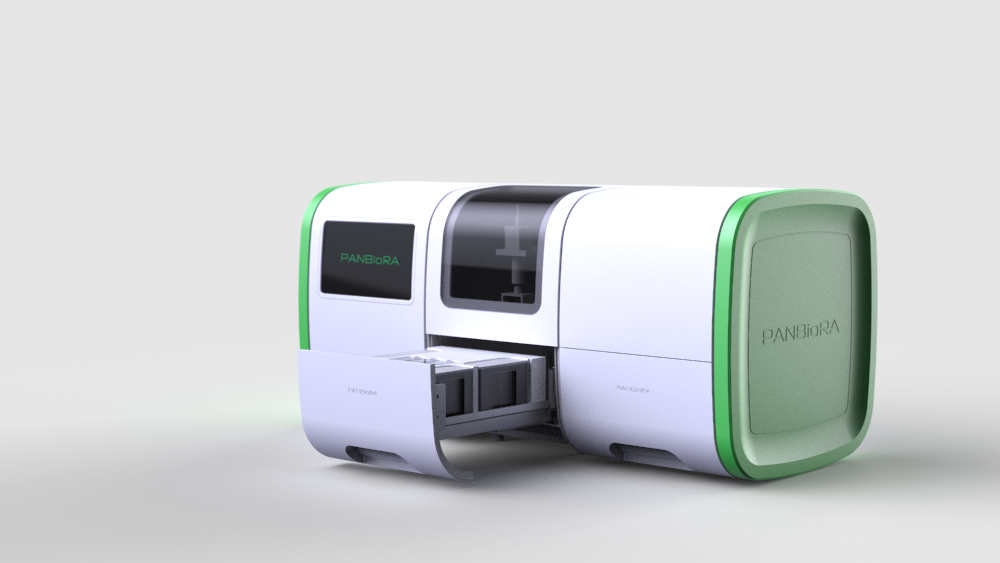
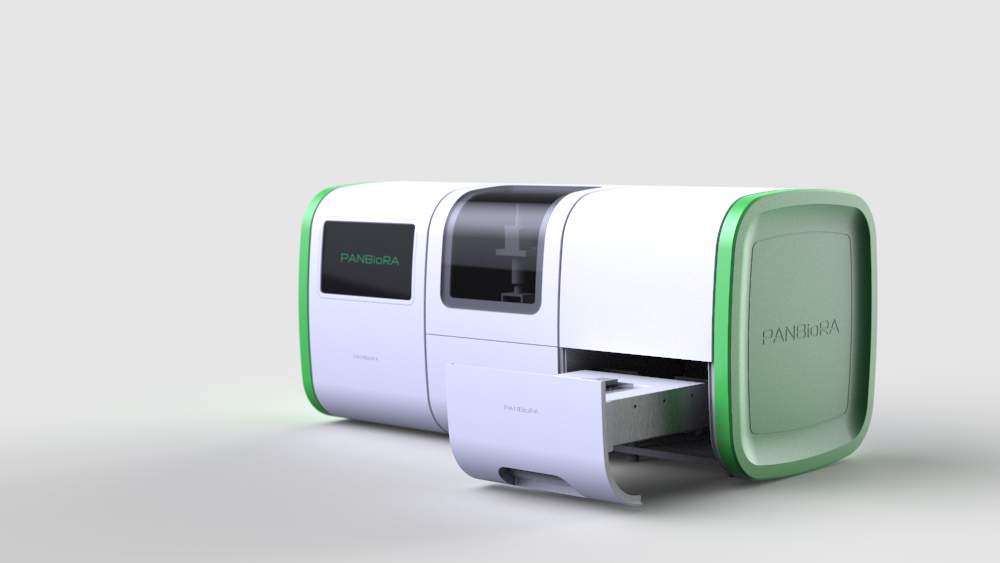
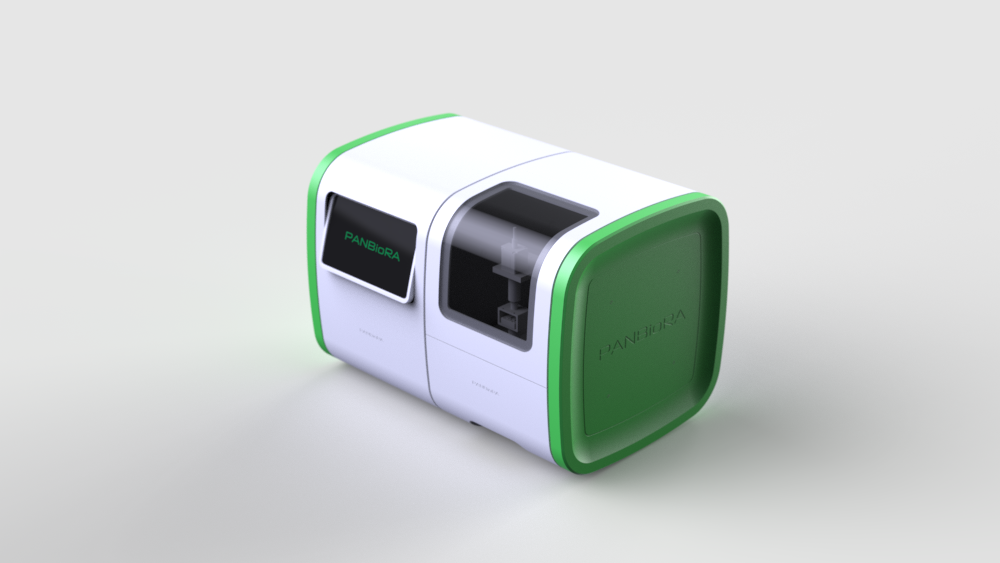
World Biomaterial Congress 2020 Workshop
Why PANBioRA?
Biomaterial-related Advances
- Compact and automated cytokine profiling
- Fast testing of genotoxicity
- Material selection – evidence of risk
- Personalised solutions and treatment options
- Integrated experimental data into the models
- Minimization of animal tests
- Significant time and cost reduction in biomaterial analysis and medical devices R&D and product development
General Advantages
- Direct application to 3D (bio)manufacturing
- Reduced risk of operator error
- Compact, easy to use, comprehensive, modular
- Access to database of previous results
- Risk monitoring during design, development and commercialisation steps
PANBioRA Numbers
The 30% reduction in cost and time for testing biomaterials alone could translate into over $ 6-30 Million savings for a single material and shorten development time by 2-3 years.

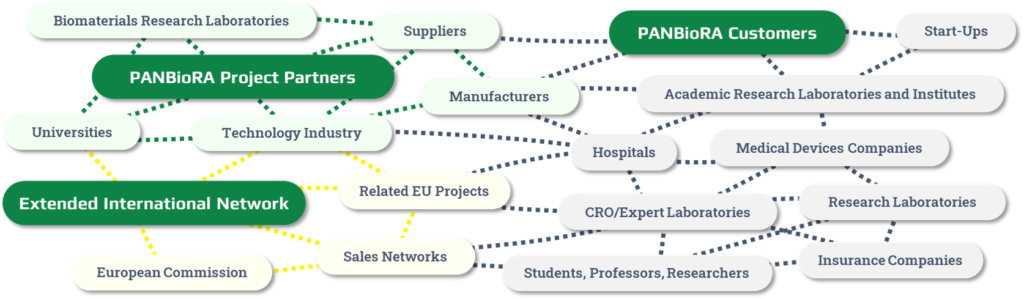
Join the PANBioRA Network